
Residual austenite
Retained austenite in weld metal is a double-edged sword; its presence and quantity have a complex and crucial impact on weld performance.Residual austenite
一. Residual austenite:What is Retained Austenite?
When discussing welds, we first need to understand continuous cooling transformation.
▶Definition: Retained austenite refers to austenite that, when steel is cooled from a high-temperature austenitic state, fails to fully transform into low-temperature phases such as bainite, martensite, or ferrite due to the influence of chemical composition and cooling rate, and remains at room temperature.
▶Formation Causes: Certain alloying elements, especially nickel (Ni), manganese (Mn), and carbon (C), significantly lower the martensite transformation initiation temperature. When the Ms point is low enough, upon cooling to room temperature, some austenite cannot transform due to insufficient driving force and is “frozen,” becoming retained austenite.
二. Residual austenite:The Dual Role of Retained Austenite in Welds
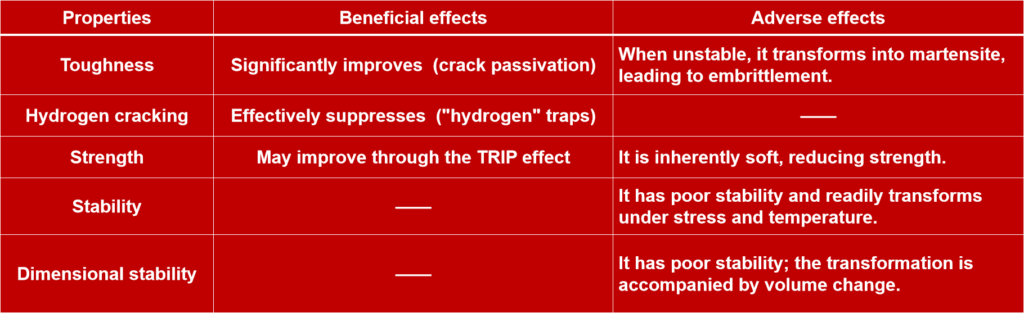
Beneficial Aspects:
❶. Improved Toughness and Crack Resistance (Especially Important)
▶Mechanism: Retained austenite is a ductile phase. In the crack propagation path, encountering ductile retained austenite can effectively passivate the crack tip and absorb energy, thus significantly improving the impact toughness (especially low-temperature toughness) of the weld metal.
▶ Application: In the design of modern high-strength steel welding materials, especially low-temperature steel welding materials and high-toughness welding materials, elements such as nickel are intentionally added to introduce a small amount (usually 3%-10%) of stable retained austenite to obtain excellent resistance to brittle fracture.
❷. Absorption of hydrogen atoms, reducing susceptibility to hydrogen-induced cracking
▶ Mechanism: Austenite has extremely high solubility for hydrogen but a very low diffusion coefficient. This means that hydrogen atoms are more easily dissolved in retained austenite and “trapped,” making it difficult for them to diffuse to potentially dangerous areas (such as grain boundaries and hard martensite lath boundaries), thereby reducing the risk of hydrogen-induced delayed cracking.
❸. TRIP effect
▶ Mechanism: In welds with certain specific compositions, retained austenite undergoes a phase transformation-induced plasticity when subjected to external force (deformation), transforming into martensite. This phase transformation process itself consumes additional energy and induces work hardening, thereby improving the uniform elongation and tensile strength of the weld.
Disadvantages:
❶. Stability Issues: Transformation into Untempered Martensite
▶ Mechanism: Retained austenite is a metastable phase. During service, if subjected to external forces, temperature changes (low temperatures), or disturbances from post-weld heat treatment, it may transform into hard and brittle untempered martensite.
▶ Consequences: This transformation can lead to:
⑴ Sharp Decrease in Toughness: Brittle martensite becomes the initiation point for microcracks.
⑵ Dimensional Instability: Martensite has a higher specific volume than austenite; the transformation can cause localized volume expansion, potentially leading to changes in component dimensions or the generation of internal stress.
⑶ In high-temperature service environments, the decomposition of unstable retained austenite can lead to performance degradation.
❷. Relatively Low Strength and Hardness
▶ In applications requiring high hardness and wear resistance (such as weld overlay materials), excessive soft retained austenite can result in insufficient surface hardness and decreased wear resistance.
❸. Corrosion Resistance Issues (For Stainless Steel Welds)
▶ In stainless steel welding, the weld microstructure is typically a duplex structure of austenite and a small amount of ferrite. Retained austenite is the dominant phase. However, improper chemical composition or cooling rate control can lead to carbide precipitation, triggering intergranular corrosion. The presence of ferrite can inhibit the precipitation of austenite grain boundary carbides, improving resistance to intergranular corrosion.
三. How to Control Retained Austenite in Welds?
The choice of welding materials and processes directly determines the content and stability of retained austenite.
❶. Chemical Composition Design (Most Critical)
▶ Austenite Stabilizing Elements: Increasing the content of elements such as Ni, Mn, C, and Cu lowers the Ms point and increases the amount of retained austenite.
▶ Ferrite Stabilizing Elements: Increasing the content of elements such as Cr, Mo, Si, and V promotes ferrite or carbide formation and reduces retained austenite.
▶ Modern high-performance welding consumables control the content and stability of retained austenite through precise “composition window” design.
❷. Welding Process Parameters
▶ Heat Input and Cooling Rate: Higher heat input (slow cooling) favors the transformation of austenite into ferrite and bainite, reducing retained austenite. Lower heat input (rapid cooling) favors the retention of austenite.
▶ Interpass Temperature: Affects the overall thermal cycle, thus influencing phase transformation behavior.
❸. Post-Weld Heat Treatment
▶ Post-weld heat treatment typically decomposes metastable retained austenite, transforming it into more stable tempered bainite or carbides, thereby reducing or eliminating retained austenite. This is crucial for improving dimensional stability and for components serving at high temperatures.
四. Summary
Key Conclusion: In welding structural steel and high-strength steel, an appropriate amount and stable amount of retained austenite is the “secret weapon” for obtaining high-toughness welds. In applications requiring high hardness, high wear resistance, or high dimensional stability, it is necessary to minimize or eliminate retained austenite. Therefore, the control of retained austenite reflects the precision and artistry of welding metallurgy in material design.

